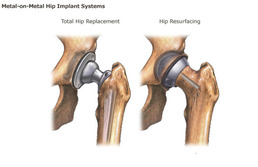
Metal-on-metal hip replacements such as the DePuy Pinnacle have been in the news* frequently because of hip lawsuits related to the devices’ alleged side effects, such as metal poisoning. The Rottenstein Law Group, a DePuy Pinnacle law firm, notes that pending Pinnacle lawsuits in federal courts are numbering almost 4,000, according to court documents in In re: DePuy Orthopaedics, Inc. Pinnacle Hip Implant Products Liability Litigation – MDL No. 2244.
The nationwide lawsuits are managed as a consolidation called “multidistrict litigation” in the U.S. District Court for the Northern District of Texas. That the number of lawsuits has reached nearly 4,000 is not a surprise to Rottenstein Law Group Principal Rochelle Rottenstein.
“On a daily basis we receive calls from those who believe their metal-on-metal hip implants such as the DePuy Pinnacle have injured them,” Rottenstein said. “It is only appropriate that the issue be decided in the court system.”
Read full story at PRWeb.com: DePuy Pinnacle Lawsuit Update: Rottenstein Law Group Notes That Number of Pending Pinnacle Lawsuits Reaches Nearly 4,000
The nationwide lawsuits are managed as a consolidation called “multidistrict litigation” in the U.S. District Court for the Northern District of Texas. That the number of lawsuits has reached nearly 4,000 is not a surprise to Rottenstein Law Group Principal Rochelle Rottenstein.
“On a daily basis we receive calls from those who believe their metal-on-metal hip implants such as the DePuy Pinnacle have injured them,” Rottenstein said. “It is only appropriate that the issue be decided in the court system.”
Read full story at PRWeb.com: DePuy Pinnacle Lawsuit Update: Rottenstein Law Group Notes That Number of Pending Pinnacle Lawsuits Reaches Nearly 4,000



 RSS Feed
RSS Feed